Major advances in our understanding of the molecular pathogenesis of myeloid malignancies like myelodysplastic syndromes (MDS), myeloproliferative neoplasms (MPNs), and acute myeloid leukemia (AML) have led to new targeted therapies and increased clinical utility of molecular testing. These advancements have prompted updates to guidelines that significantly influence clinical practice, including diagnosis, risk stratification, and therapy selection1.
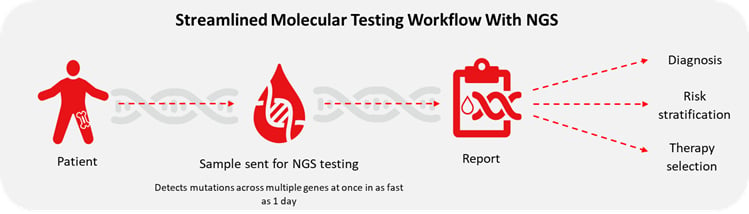
To fulfill its promise of improved outcomes, the precision medicine paradigm requires a molecular analysis–informed approach to frontline patient care. In the past, clinicians were forced to make first-line therapy decisions without key molecular insights because obtaining those results could take up to several weeks. When the results finally arrived, they would re-evaluate and potentially switch the patient to a more effective therapy. Unfortunately, for patients in health crisis mode, getting the right therapy right away can make all the difference.
Rapid NGS can generate a complete mutational profile across several genes at once in as fast as a single day. Advances in workflow automation accelerates speed from sample to report and empowers clinicians to make rapid biomarker-informed decisions on the frontline of patient care, potentially improving outcomes.
Hear Dr. Bekim Sadikovic share how his lab has implemented next generation sequencing (NGS) as a frontline testing method for assessing patients with suspected myeloid malignancies. He reviews recent data from his lab and discusses the clinical utility of upfront molecular profiling for acute myeloid leukemia (AML), myelodysplastic syndromes (MDS), and myeloproliferative neoplasms (MPN).

Bekim Sadikovic, PhD, DABMG, FACMG
Professor, Research Chair, and Program Head, Western University and London Health Sciences, Ontario, Canada

Giovanni Insuasti, MD
Assistant Professor of Pathology and
Medical Director of Molecular Oncology
Atrium Health Wake Forest Baptist Medical Center,
North Carolina
Listen to Dr. Insuasti provide clinical background on the diagnostic workup of myeloid neoplasms and share how his lab was able to improve the turnaround time for myeloid genomic profiling using automated in-house NGS.
As the number of clinically relevant biomarkers continues to expand across many genes, traditional sequential single-analyte testing is becoming less sustainable.
Analyzing targets individually is impractical, inefficient, and far too time-consuming. Given its ability to profile many genes simultaneously, NGS is now routinely used in the molecular workup of myeloid malignancies. Yet still, many platforms feature long, complex workflows and require large sample numbers for cost efficiency, all of which can delay results for weeks. And if multiple single-analyte tests are employed up front, the sample may be depleted entirely before NGS can be performed and critical molecular insights gleaned.2


David M. Swoboda, MD
Tampa General Hospital Cancer Institute, Tampa, Florida
"Critical, as we move forward into this era of precision oncology in AML, is our ability to perform rapid next-generation sequencing to identify therapeutic molecular targets in AML. Understanding the disease’s genetic makeup in AML will allow us to provide the best care and clinical outcomes while also maximizing the utilization of our hospital resources.”

Dr. Kojo Elenitoba-Johnson
Perelman School of Medicine, University of Pennsylvania
“There are several protocols, including extensive genetics and immunophenotyping, which are required to establish the appropriate approach to treat patients. The sooner we can get results and deliver treatment, the better the outcomes.”

Dr. Giovanni Insuasti
Atrium Health Wake Forest Baptist Medical Center, North Carolina
“Faster turnaround times have been a breakthrough for our institution. If you talk to our clinicians, they are extremely happy because reporting went from 2-3 weeks to 2-3 days on average, which has been really good for patients.”
Recent updates to clinical guidelines support the need for more rapid and comprehensive molecular profiling of myeloid malignancies.
For example, the European LeukemiaNet (ELN) guidelines for AML now include an expanded list of relevant biomarkers for molecular testing at time of diagnosis and the addition of rapid turnaround times for key targetable and diagnostic mutations.
References
1. Döhner H, Wei AH, Appelbaum FR, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140(12):1345-1377.
2. Sande CM, Wu R, Yang G, et al. Rapid and automated semiconductor-based next-generation sequencing for simultaneous detection of somatic DNA and RNA aberrations in myeloid malignancies. J Mol Diagn. 2023;25(2):87-93.
We've detected your location to be Japan.
Sorry, you cannot access this website. The content on www.oncomine.com is only intended for healthcare professionals. Formore information on our research solutions, please visit ThermoFisher.com
このウェブサイトは、日本国内の医療関係者の方への情報提供を目的としており、一般の方に対する情報提供を目的としたものではないことをご了承ください。研究用製品の情報はThermoFisher.comよりご覧ください。