Supporting independent research projects in order to promote excellence in molecular diagnostics.
Supporting independent research projects in order to promote excellence in molecular diagnostics.
Proposals will be accepted through the submission portal during open submission period. Alternatively we may accept via the email medical.affairs@thermofisher.com. Please visit this web page during the open submission period to submit your application.
Read our blog for tips on how to submit a strong grant application.
Oncomine Grants Clinical Research Program overview
The Oncomine Clinical Research Grant Program supports investigator-initiated studies (IIS) and education projects, on molecular profiling in oncology, with the goal to increase high quality molecular profiling and to help democratize the future of precision medicine.
Proposal reviews are conducted by independent and internationally recognized experts together with Thermo Fisher Scientific medical and scientific leadership. Funding is considered based on scientific merit.
The application process is managed by the Oncomine Clinical Research Grant Office, part of the Medical Affairs team of the Clinical Sequencing Division at Thermo Fisher Scientific. For detailed information about how to apply, including documentation requirements, review process, and decision notification, refer to the Guidelines for Applicants.
Grant applications should be submitted electronically (English only) using the specific Proposal Form. Proposals should outline the planned activities to be performed within a maximum of 12 months. A budget is required for every grant request and should detail the proposed use of requested funds. The maximum budget request for each project is set at $200,000.
More Information:

“The Oncomine Grant program gives a chance to those who wouldn’t otherwise have it to be able to work with next-generation sequencing technologies. The funding from the Oncomine Grant program can really help us overcome one of the biggest hurdles for smaller and less funded centers to be able to work with these types of emerging technologies.”
Brandon Sheffield, MD
William Osler Health System, Canada
2020 Oncomine Clinical Research Grant awardee
“Industry funding [like the Oncomine Clinical Research Grant program] is important because it can generate a sort of virtual circle, creating a link between the industry and different academic institutions and hospital research labs, and this link can foster the development and the implementation of new platforms or new assays focused on the unmet clinical needs of the future.”
Elena Guerini Rocco, MD
European Institute of Oncology IRCCS, Italy
2021 Oncomine Clinical Research Grant awardee


“The opportunity the Oncomine Grant has afforded us to work on the analysis of the
T cell repertoire has opened also a number of opportunities for my team to collaborate
on larger studies. We are now moving from the analysis that we have done with the Oncomine Grant to a large study at the national level to further evaluate these biomarkers and the T cell repertoire.”
Elin Gray, PhD
Edith Cowan University, Australia
2021 Oncomine Clinical Research Grant awardee
The evaluation process is performed by a committee of external experts in the field, along with Thermo Fisher Scientific medical and scientific leadership. The committee selects awardees based on the scientific merit of the proposed project.
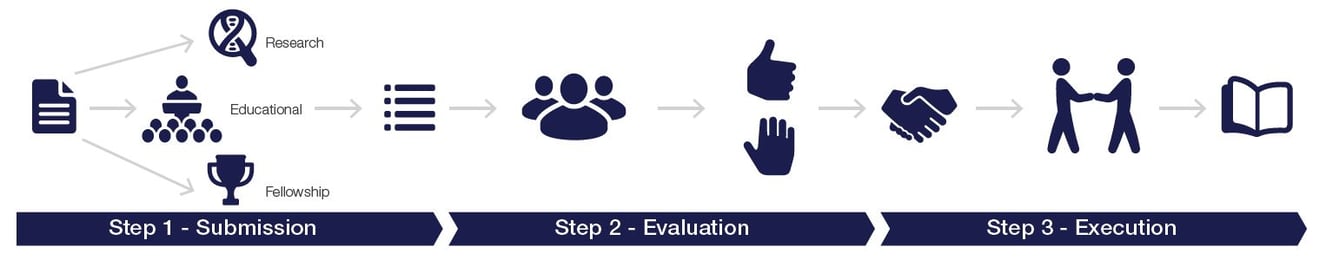
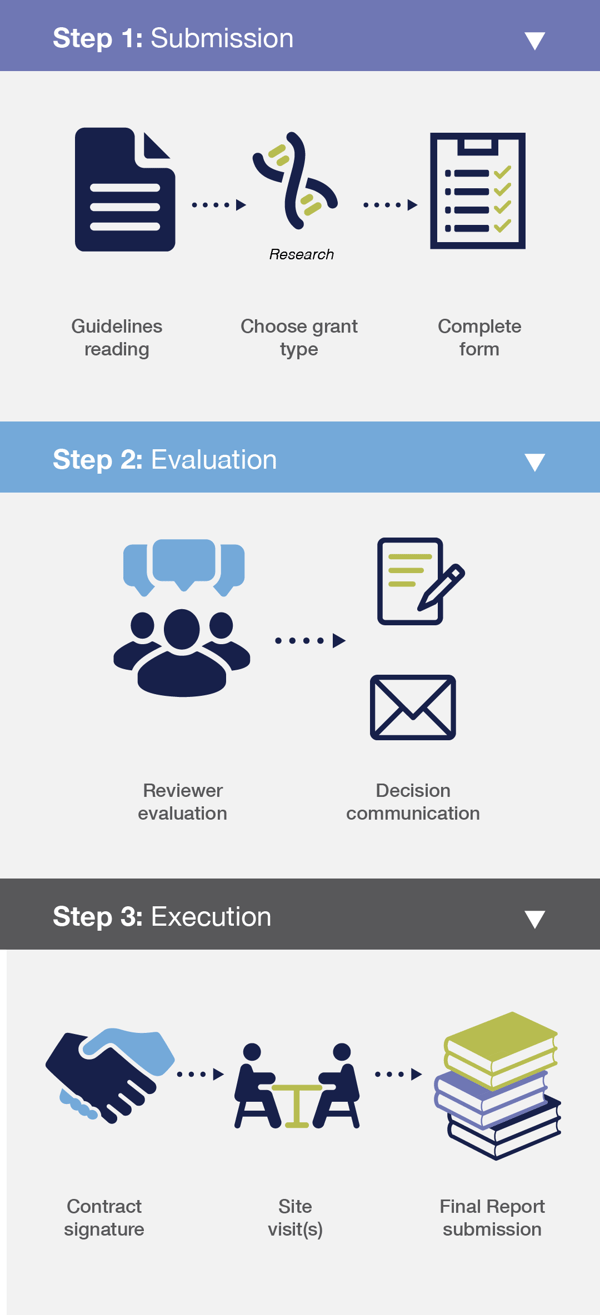
Step 1: Submission
Submission is done by sending the proposal form to the following email address: medical.affairs@thermofisher.com or using the online grant management portal.
Step 2: Evaluation
Submissions are confirmed by the grant office via email. Decisions are communicated 6 to 8 weeks after the closing date of the call.
Step 3: Execution
Upon contract signature, the requested support will be granted. The grant office team will continue to support the development of the awarded project. A final report is due to the grant office team within the agreed time frame.
We thank all applicants that submitted projects across our 2023 calls for applications.
We are pleased to announce the 2023 grant awardees below.

“Getting melanoma diagnosis right: migration and clinical validation of the MIA genomic ancillary test on the Genexus platform for regular clinical testing”

“Biomarker Assessment by Next Generation Sequencing Using the Supernatants from Fine Needle Aspiration of NSCLC”


"Integrated molecular analysis to depict signatures associated with TKI treatment response in advanced NSCLC and to identify novel predictive biomarkers”
We've detected your location to be Japan.
Sorry, you cannot access this website. The content on www.oncomine.com is only intended for healthcare professionals. Formore information on our research solutions, please visit ThermoFisher.com
このウェブサイトは、日本国内の医療関係者の方への情報提供を目的としており、一般の方に対する情報提供を目的としたものではないことをご了承ください。研究用製品の情報はThermoFisher.comよりご覧ください。