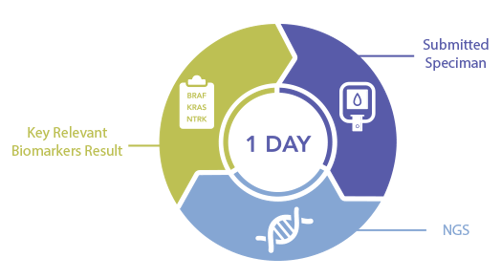
Oncomine Precision Assay on the Ion Torrent Genexus System provides a complete NGS testing workflow that lets every lab go from specimen to report in a single day*. Now your laboratory can produce NGS results in the same time as testing for single-gene biomarkers like PD-L1.
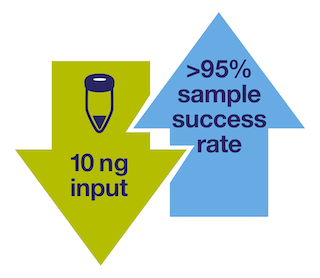
Did you know that some NGS send-out labs have test failure rates of 20% due to high input requirements?
At that rate, 1 out of 5 samples do not receive a result, and many more are not tested at all due to lack of tissue availability.
Not with Oncomine Solutions. You can experience a sequencing success rate of over 95% because we only require 10ng of nucleic acid input. That means you can get an NGS result for more of the precious samples you have.
Get Results in a Day
Get a profile for 50 key genes in one day. See the complete picture by combining results for single-gene biomarkers like PD-L1 with other important biomarkers like BRAF, KRAS, ALK, NTRK, ROS1, and RET among others.
Get Results for More of Your Samples
Oncomine Solutions offers the lowest sample input requirement of any NGS platform. Now you can submit more of your samples for testing and no longer need to deal with high test failure rates, even when tissue is very limited.
Enjoy the Simplicity
Get a clear and concise report that presents all relevant variants with annotated information about targeted therapies, medical guidelines, and enrollment in ongoing clinical trials.
WCLC 2020 Product Theatre On-Demand:
Like many other technologies, next-generation sequencing (NGS) has undergone massive innovation and transformation. But there is still a lot of misinformation and misconception: for example, that it “takes weeks to get results,” or that “large samples are required,” or it’s “very complex.” Don't believe it!
Watch our presentation to:
References:
1. Next-Generation Sequencing in 305 Consecutive Patents: Clinical Outcomes and Management Changes; Gregory J. Kubicek, MD., et al., Journal of Clinical Oncology, Aug. 2019.
2. J Clin Oncol. 2019 Apr 20;37(12):992-1000. doi: 10.1200/JCO.18.01042. Epub 2019 Feb 20.
3. Clinical utility of FoundationOne tissue molecular profiling in men with metastatic prostate cancer; Jason Zhu, et al.; Urologic Cancer: Seminars and Original Investigations, July 2019.
4. Abstract 4889: Comparison of tumor mutational burden using the Ion Oncomine™ TML and FoundationOne™ assays with routine clinical FFPE tissue samples to predict durable clinical benefit in lung cancer and melanoma patients - a multivariate analysis integrating PD-L1 and CD8+ evaluation. Heeke S. et al. DOI: 10.1158/1538-7445.AM2019-4889; July 2019.
For Research Use Only. Not for use in diagnostic procedures. *Specimen-to-report workflow will be available after the Ion Torrent Genexus Purification System and integrated reporting capabilities are added in 2020.
We've detected your location to be Japan.
Sorry, you cannot access this website. The content on www.oncomine.com is only intended for healthcare professionals. Formore information on our research solutions, please visit ThermoFisher.com
このウェブサイトは、日本国内の医療関係者の方への情報提供を目的としており、一般の方に対する情報提供を目的としたものではないことをご了承ください。研究用製品の情報はThermoFisher.comよりご覧ください。