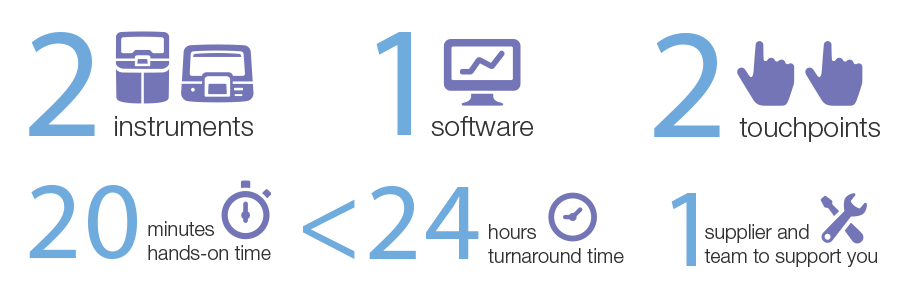
Whilst other NGS workflows are advertised as “end to end” and “automated”, we believe that the Ion Torrent™ Genexus™ System and assay menu represents the only true end to end solution in the market. 2 instruments, 1 software and assays, all from one supplier will enable you go from biological specimen to report in as little as 1 day, involving only 20 minutes of hands on time.
Enjoy our on-demand webinar series that gives you a closer look at the new Genexus System and FusionSync technology.








Not with the Genexus System. Watch video below to learn how bringing NGS in-house is easier than you think.
Read the latest news and user stories about how the Genexus System is advancing precision oncology in our quarterly e-news.
Latest News
In this issue, we present a new virtual demo of our Genexus System. Thinking about how the Genexus System works and how it could fit into your lab? In our virtual laboratory environment powered by augmented reality (AR) technology, you can see...Read more.

Are you worried about NGS bioinformatics? Using different software and requiring an expert to stich it all together? No need anymore! The Genexus Software, one secure, streamlined and easy-to-use NGS analysis software, provides a seamless experience from end to end, starting with entering sample data and finishing with report.
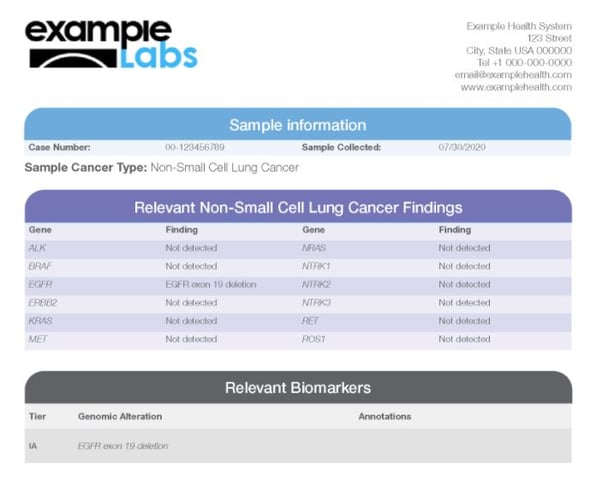
The Oncomine Reporter delivers clear and concise reports that present all relevant variants with annotated information about targeted therapies, guidelines, and enrollment of ongoing clinical trials.
The report is customizable so that you can tailor it to fit your team's needs.
Learn More About Oncomine Informatics
A molecular biology laboratory at the Hospital Universitari Vall d'Hebron performs NGS analysis with the Genexus System.
The Oncomine Precision Assay on the Ion Torrent Genexus System detects low-level variants that are particularly important for liquid biopsy testing. It provides:

Although tissue samples are still the gold standard for molecular profiling, there exist many limitations.
Tissue can be inaccessible or there may not be enough material to sequence. Further, obtaining a tissue sample is difficult to repeat and not useful for monitoring and the sample may not reflect tumor heterogeneity. Liquid biopsy may be a viable alternative to tissue sequencing, as it is minimally invasive, provides a comprehensive tumor profile, and allows for repeat sampling.
With the Oncomine Precision Assay, you have the flexibility to sequence standard tissue or minimally invasive plasma samples, from one test on a single workflow.

Dr. Nicola Normanno and Dr. Philip Jermann on How The Genexus System Can Fit in NGS Experienced or Beginner's Lab. October 2019
References:
1. Next-Generation Sequencing in 305 Consecutive Patents: Clinical Outcomes and Management Changes; Gregory J. Kubicek, MD., et al., Journal of Clinical Oncology, Aug. 2019.
2. J Clin Oncol. 2019 Apr 20;37(12):992-1000. doi: 10.1200/JCO.18.01042. Epub 2019 Feb 20.
3. Clinical utility of FoundationOne tissue molecular profiling in men with metastatic prostate cancer; Jason Zhu, et al.; Urologic Cancer: Seminars and Original Investigations, July 2019.
4. Abstract 4889: Comparison of tumor mutational burden using the Ion Oncomine™ TML and FoundationOne™ assays with routine clinical FFPE tissue samples to predict durable clinical benefit in lung cancer and melanoma patients - a multivariate analysis integrating PD-L1 and CD8+ evaluation. Heeke S. et al. DOI: 10.1158/1538-7445.AM2019-4889; July 2019.
For Research Use Only. Not for use in diagnostic procedures.
We've detected your location to be Japan.
Sorry, you cannot access this website. The content on www.oncomine.com is only intended for healthcare professionals. Formore information on our research solutions, please visit ThermoFisher.com
このウェブサイトは、日本国内の医療関係者の方への情報提供を目的としており、一般の方に対する情報提供を目的としたものではないことをご了承ください。研究用製品の情報はThermoFisher.comよりご覧ください。