As in-house next-generation sequencing (NGS) testing becomes more prevalent, more hospitals are evaluating the impact of moving away from the test send-out model. Beyond the clinical benefits from the faster turnaround time of in-house testing, there are also a number of ways that hospitals can realize economic advantages.
Here are five key advantages that oncologists, hospital administrators, and lab directors consider when evaluating the future of multi-biomarker testing at their institutions.
Clinical oncologists in community hospitals observe that a noticeable percentage of patients are lost when they seek a second opinions. Ensuring that patients have rapid return of all testing results before a second opinion visit—including a comprehensive biomarker report —helps oncology
increase retention.
When a patient receives the same testing options and treatment choices from a second opinion source as they did from their community hospital, they are more likely to return to their community hospital for their care.
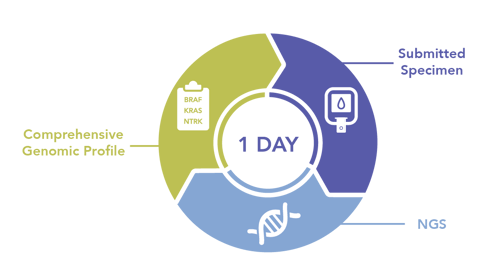
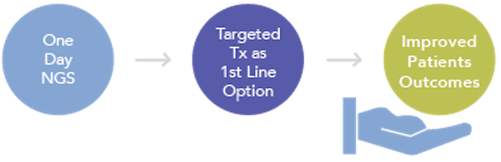
In-house NGS testing enables labs to return results in as fast as one day, which provides oncologists a comprehensive genomic profile in a timeframe that allows them to select the most appropriate first line therapy from all available options. Both targeted and immune therapies can be routinely considered, depending on the particular genomic profile.
The most appropriate first line therapy can increase the likelihood of good clinical outcomes for patients, who remain within the health system for continued care as their disease is managed over its course.
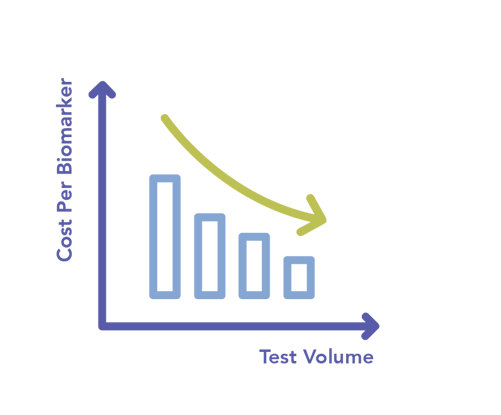
As pharmaceutical companies expand the number of available targeted therapies, the demand for multi-biomarker testing will continue to grow.
Compared to single-gene methods, NGS testing is a more cost-effective approach for scaling multiplex test volume over time. CLIA labs that adopt in-house NGS now will be much better suited to minimize cost, conserve tumor tissue, and grow testing revenue from multi-biomarker testing well into the future.
NGS testing is no longer limited to leading-edge teaching hospitals and oncology centers. Thanks to technology advancements, the testing workflow is so simple that molecular labs can bring it in-house without having to recruit
and train experts.
This operational efficiency enables hospitals to provide a strong precision oncology offering, while maintaining the resource and labor flexibility to focus on other priorities.

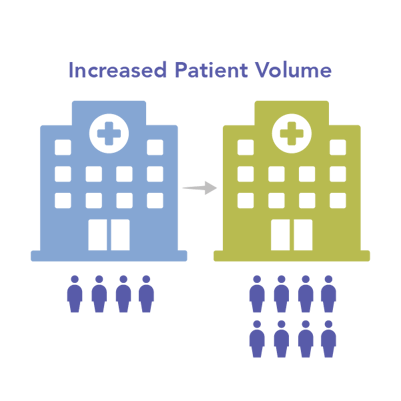
Precision medicine offers a marketing and brand differentiator for hospitals. By supplying the latest technology for genomic profiling and delivering results in a timeframe that helps optimize therapy selection and outcomes, hospitals with in-house NGS provide a clear advantage over other institutions.
Strengthening the hospital brand can ultimately help drive increased patient volume.
NGS in routine NSCLC biomarker testing
This CAP TODAY webinar explains how to make NSCLC biomarker testing a routine part of your care process.
NGS for Myeloid Malignancy Profiling
Dr. Michael Rauh from Queen's University discusses the role and impact of NGS for profiling myeloid malignancies such as AML and MDS.
References:
1. Clinical applicability and cost of a 46-gene panel for genomic analysis of solid tumors: Retrospective validation and prospective audit in the UK National Health Service; PLOS Medicine | DOI:10.1371/journal.pmed.1002230 February 14, 2017.
2. Clinical utility of reflex testing using focused next generation sequencing for management of paitents with advanced lung adenocarcinoma; Miller TE, et al. J Clin Pathol 2018;0:1-7. DOI:10.1136/jclinpath-2018-205396.
3. *NSCLC example: NCCN Guidelines v13,2017, Metastatic Disease
4. Multiple biomarker tissue consumption and completion rates with single-gene tests and investigational use of Oncomine Dx Target Test for advanced non-small-cell lung cancer: A single-center analysis. Tiany M. Yu, et al. Clinical Lung Cancer, Vol. -, No. -, 1-10a 2018 The Author(s). Published by Elsevier Inc.
5. Marchetti et al. 2018. https://doi.org/10.1016/j.cllc.2018.05.012
We've detected your location to be Japan.
Sorry, you cannot access this website. The content on www.oncomine.com is only intended for healthcare professionals. Formore information on our research solutions, please visit ThermoFisher.com
このウェブサイトは、日本国内の医療関係者の方への情報提供を目的としており、一般の方に対する情報提供を目的としたものではないことをご了承ください。研究用製品の情報はThermoFisher.comよりご覧ください。