In his recent talk at OncomineWorld 2023, Dr. Bekim Sadikovic presented a strong, evidence-based argument for frontline next-generation sequencing (NGS) in myeloid malignancy testing.

Bekim Sadikovic PhD, DABMGG, FACMG
Professor, Research Chair, and Molecular Program Head
Western University and London Health Sciences
Ontario, Canada
He began with an introduction to the LHSC Mol Dx Program / Verspeeten Clinical Genome Centre, describing how they’ve implemented NGS as a standard tier-1 test for suspected hematological malignancies. Next, Dr. Sadikovic briefly discussed their validation of a targeted NGS myeloid panel and highlighted their NGS patient registry, results, and the clinical impact of upfront NGS testing for their first 3,500 patients.
NGS has a high diagnostic yield across various myeloid malignancies
The NGS advantage is clear regarding its coverage of all the common myeloid mutations in the NCCN guidelines, including fusions. In validation, their targeted NGS assay demonstrated 100% specificity, 95.5% sensitivity, and 94.2%-98.1% concordance in an interlaboratory comparison study.
After NGS was implemented in clinical practice, Dr. Sadikovic assessed the initial impact of their ‘genotype first’ approach to testing all suspected myeloid cancer cases. Of the first 380 patients tested, 189 had reportable variants giving a diagnostic yield of 49.7%. Of the remaining patients where a reportable variant was not identified, most did not have a myeloid malignancy.
To further evaluate the clinical value of frontline NGS, a second study was conducted after 2 years of testing using data from 1613 patients. The overall diagnostic yield for this patient cohort was 43%, with high diagnostic yields across the various types of myeloid malignancies, including acute myeloid leukemia (AML), myelodysplastic syndromes (MDS), and myeloproliferative neoplasms (MPN). These high diagnostic yields support NGS as a valuable tool for providing key insights that could transform patient management.
NGS can identify key prognostic markers beyond what is traditionally available with other methods
Next, Dr. Sadikovic dove deeper into his findings to identify the specific prognostic implications of having NGS results available at the time of diagnosis for AML, MDS, and MPN. He shared the following data to explain the clinical utility across myeloid malignancies:
| Clinical utility of frontline NGS for AML, MDS, & MPN |
AML:
Key Takeaway: NGS profiling allows for the identification of critical prognostic markers beyond what is typically available with karyotyping alone. |
MDS:
Key Takeaway: NGS profiling provides mutational data beyond our standard scoring systems using cytogenetics that could lead to improved patient risk stratification. |
MPN:
Key Takeaway: NGS can identify additional co-occurring variants beyond JAK2 mutations, which can help to better predict prognosis and leukemic transformations in these patients. |
Overall, it was concluded that having NGS results upfront was valuable across cancer types for providing important insights that allowed for better determination of patient prognosis and risk classification.
NGS can detect co-occurring variants at the time of diagnosis that may impact clinical outcomes
Looking at the entire data set, Dr. Sadikovic noted that mutational co-occurrence is common in hematological malignancies. He went on to specify that certain patterns are co-related with specific subtypes (depicted in the circos plots below).
The presence of co-occurring variants has been reported to impact the course of disease, inform risk stratification, and are predictive of clinical outcomes (eg, those with triple-mutated AML have inferior survival). He concluded that NGS can efficiently identify mutational co-occurrence and provide additional information that would not be readily available using single-gene methods.
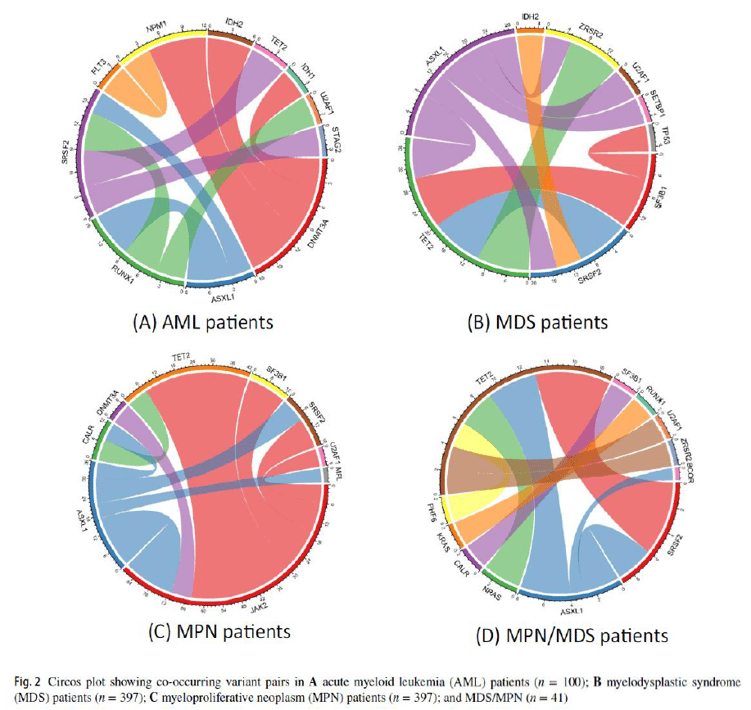
Circos plots above show co-occuring variant pairs across disease types. Each variant has an associated color and thickness of the bands indicates the frequency that a specific variant pair was identified.
Frontline NGS has the potential to reduce test redundancy
Lastly, Dr. Sadikovic’s analyzed the concordance of NGS with FISH and karyotyping for fusion detection. Within his patient cohort:
• 37/38 patients were positive for fusions by both FISH and NGS; 1 patient was missed by FISH.
• 39/45 patients were positive for translocations resulting in fusions by both karyotyping and NGS; 6 patients were missed by karyotyping.
These findings indicate that NGS can identify some fusions that may not be detectable by other methods due to low frequency or other technical issues. The high concordance of NGS with FISH and karyotyping, underscores the opportunity to improve test utilization. When combined with a morphologic triage protocol, frontline NGS could reduce testing redundancy.
Dr. Sadikovic concluded that ample evidence demonstrates the feasibility and potential clinical utility of integrating an NGS-first approach for patients with hematologic malignancies.
Key Takeaways on the value of an NGS-first approach for hematological malignancies:
• Enable an increased diagnostic yield across various myeloid malignancies
• Can identify critical prognostic molecular biomarkers that would not typically be available with traditional methods like cytogenetic karyotyping
• Can detect co-occurring mutations that may impact the course of the disease, risk stratification, and clinical outcomes
• Has the potential to reduce test redundancy with appropriate triaging
Precision medicine is rapidly changing our understanding of cancer research and treatment decisions. These breakthrough, personalized treatments hold promise even for patients with historically hard-to-treat diseases, like lung or breast cancer. But
In his recent talk at OncomineWorld 2023, Dr. Bekim Sadikovic presented a strong, evidence-based argument for frontline next-generation sequencing (NGS) in myeloid malignancy testing.
Bekim Sadikovic PhD, DABMGG, FACMGProfessor, Research Chair, and...
In a recent Association for Molecular Pathology (AMP) workshop, Dr. Cecilia Yeung shared how her lab is implementing rapid next-generation sequencing (NGS) to help advance hemato-oncology research and expand access to underserved populations.
...