Comprehensive genomic profiling (CGP) is one of the latest innovations in next-generation sequencing (NGS) that offers unprecedented breadth and depth of sequencing across multiple biomarkers.
CGP spans a variety of genomic alterations (copy number gains and losses, fusions/translocations, insertions, deletions, and single base substitutions), detects genomic instability through molecular deficiency pathways, and identifies complex biomarkers—all in 1 assay.
With CGP, it’s possible to identify relevant cancer biomarkers established in guidelines and clinical trials and empower clinicians with the genomic insights they need to make precision oncology a reality.
Cancer biomarkers and companion diagnostics enable tailored treatment for a stronger, more strategic defense in the battle against cancer. Identifying these clinically actionable biomarkers fast and cost-efficiently is critical to realizing the promise of precision medicine.
For cancers with multiple associated targeted therapies, CGP allows for the simultaneous assessment of all relevant biomarkers with minimal input material.
In contrast, single analyte testing of multiple biomarkers requires more tissue and may result in increased costs, depending on the number of biomarkers profiled. For certain cancers of known primary origin, NGS that employ smaller panels may also be a suitable choice.
When the primary cancer and its drivers are unknown, or when it may involve complex biomarkers, the broad sequencing power of CGP can deliver critical insights.
One of the biggest strengths of CGP is accessing complex biomarkers, including genomic instability. Commercially available CGP testing solutions report on tumor mutational burden (TMB), microsatellite instability (MSI), and homologous recombination deficiency (HRD).
While initial clinical research assessing tumor mutational burden (TMB) looked promising as a predictive marker for immune checkpoint inhibitor therapy, it hasn’t held up to clinical scrutiny. As such, current NCCN guidelines continue to suggest using PD-L1 immunostaining for immune checkpoint therapy stratification.
Genomic instability is a hallmark of cancer, commonly due to the inability of cancer cells to repair DNA breaks. A deficiency in the homologous recombination repair (HRR) has been associated with several tumor types, including breast, ovarian, prostate, and pancreatic cancers2. This deficiency is known as homologous recombination deficiency (HRD).
Detecting genomic instability is important for precision medicine because specific oncology therapeutics like polyadenosine diphosphate [ADP]-ribose) polymerase (PARP) inhibitors and other DNA repair-targeting drugs can induce synthetic lethality in those cells and eradicate the tumors. HRD is associated with improved prognosis (progression-free survival and overall survival), platinum sensitivity (PS), and PARP inhibitor response in high-grade ovarian cancer3.
Traditionally, detecting genomic instability has been driven by BRCA1 and BRCA2, the key genes involved in the biological pathway for homologous recombination-based double-strand break repair in the DNA.
Today, different laboratories approach HRD identification with different assays. Some assays focus only on the causes (BRCA1 and BRCA2 mutations). In contrast, others focus on the consequences (genomic instability metrics, eg, gLOH), and still others approach it from a broader perspective (CGP).
Consequently, there is no standard for interpreting the results, and considerable confusion about how it impacts treatment decisions, reflects outcomes, and treatment response.
Thermo Fisher Scientific participates in The Friends of Cancer Research Homologous
Recombination Deficiency (HRD) Harmonization Project is a global consortium engaged in improving alignment across HRD tests.
“Harmonization regarding measurement and use of HRD as a predictive biomarker will help ensure patients receive the best treatment...”
Jeff Allen, President, and CEO, Friends of Cancer Research4
In cases of advanced-stage cancer and clinically compromised patients, there’s an urgency to initiate treatment immediately. Unfortunately, initial treatment decisions made without genomic insights may not be optimal and can result in poor clinical outcomes.
A recent retrospective study of 525 newly diagnosed stage IV NSCLC patients harboring actionable oncogenic drivers reveals that genomic profiling-directed therapy may improve patient outcomes.1
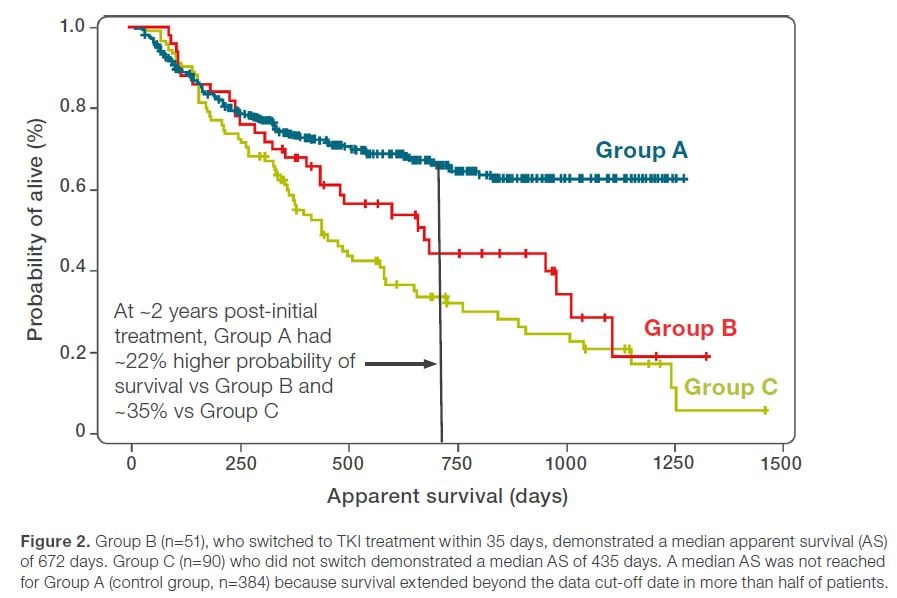
The findings suggest that treatment outcomes were significantly compromised in patients who started treatment before their genomic profiling results were reported compared to patients whose treatments were initiated after their genomic profiling results were received.
CGP solutions offering rapid results have the potential to enable first-line implementation of precision medicine therapeutics—the right medicine at the right time for the best possible outcomes.
“CGP will push the boundaries, but we cannot think about the future without thinking about the present. There are patients that need to have fast answers today.”
Luca Quagliata, PhD, BCMAS, Vice-President and Global Head of Medical Affairs, Clinical NGS and Oncology Division, Thermo Fisher Scientific
The most apparent advantage of in-house CGP is faster results to inform rapid precision medicine treatment decisions.
The more subtle value to the participating institutions will be the deep integration of their lab and clinical teams—lab directors, biostatisticians, and oncologists collaborating to coordinate each patient’s optimal treatment.
In addition, the wealth of data accumulated from CGP will continue to inform their molecular tumor boards and springboard research.
Unfortunately, many of the current CGP solutions have shortcomings that limit their utility, including incomplete profiling, inadequate sample success rates, slow turnaround times, complex workflows, and challenging bioinformatics.
To be viable, it is clear that emerging CGP solutions will need to feature very low sample requirements across all sample types, vastly improved turnaround times, automated, streamlined workflows with minimal touchpoints, and end-to-end bioinformatics with annotated reporting.
As when NGS was introduced, it’s anticipated that top academic centers and larger hospital networks will adopt CGP first. Eventually, CGP is expected to become ubiquitous across clinical settings empowering clinicians with insights for precision medicine-guided first-line treatment decisions. The promise of precision oncology will finally be fulfilled.
References:
1Smith RE, et al. Journal of Clinical Oncology (2022), doi: 10.1200/JCO.2022.40.16_suppl.1530
2https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8914493/
3https://ascopubs.org/doi/full/10.1200/PO.20.00393
4https://www.drugdiscoverynews.com/in-search-of-hrd-harmony-14880
A recently published article by the BLOODPAC Consortium details the potential of liquid biopsy in the management of cancer and highlights the barriers to adoption, particularly in underserved populations.
Cancer is one of the leading causes of...
Precision medicine is rapidly changing our understanding of cancer research and treatment decisions. These breakthrough, personalized treatments hold promise even for patients with historically hard-to-treat diseases, like lung or breast cancer. But
Dr. Eric Vail is Director of Molecular Pathology, Cedars-Sinai Medical Center,Los Angeles, California
Can you please introduce yourself and your laboratory?
I'm the director of the Cedar Sinai Medical Center Molecular Pathology Laboratory, which...