Three critical factors impact clinical actionability and patient outcomes
Tumor tissue requirements
Lower requirements means that more patients can get actionable results.
Testing turnaround times
Complete biomarker results should be available in days, not weeks.
Adequate biomarker coverage
Testing should include relevant biomarkers based on current science
Introducing the Oncomine Dx Target Test to tackle all these factors and get the best solution for your patients
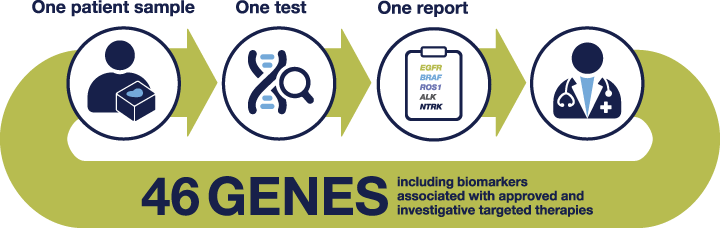
The Oncomine Dx Target Test is the first targeted next-generation sequencing (NGS) in vitro diagnostic test, which can analyze all key biomarkers for EGFR, ALK, BRAF, ROS1 kinase inhibitors, and many more currently in clinical trials
• The tissue input requirement is low, significantly lower than other CDx
• Time: The laboratory workflow only takes 4 days
• Coverage: Detects 46 gene cancer driver variants
The Oncomine Dx Target Test implemented in your hospital will enable you and your care team to provide the top care according to the guidelines, without being dependent on third-party laboratories.
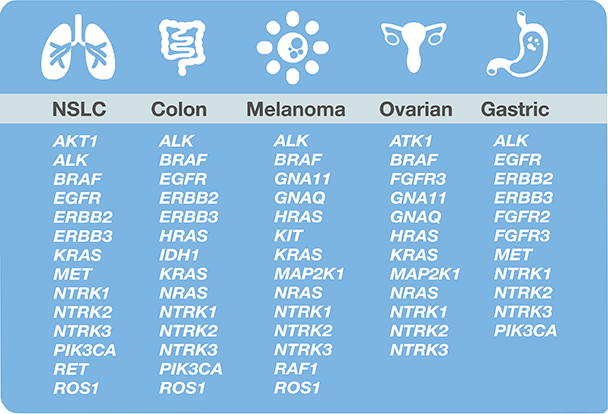
Unique coverage of biomarkers relevant for approved and investigative therapies
The Oncomine Dx Target Test detects 46 cancer driver genes, including all key biomarkers for EGFR, ALK, BRAF and ROS1 kinase inhibitors.
It can also detect many more that are currently associated with clinical trials, such as EGFR mutations including L858R, T790M and Exon 19 deletions, KRAS, ERBB2, and MET Exon 14 skipping mutations, and RET, and NTRK1/2/3 fusions
All of this can be achieved from one sample using a 4-day workflow.
-

1) Complete biomarkers results for more patients
External labs, such as Foundation Medicine, offer a single panel with biomarkers for every type of cancer. Why test for hundreds of genes that aren’t actionable for your patient?
In 46% to 80%1 of patient cases, the samples are simply too small for large panels.2 This testing approach may require invasive re-biopsies or lead to sub-optimal therapy.
The Oncomine Dx Target Test is able to return a full biomarker report for NSCLC from much smaller tumor samples, benefitting more patients.
When it comes to tissue sample, how much is required for testing? See data from labs around the world. View Infographic »
-

2) Reduced cost of care
Outsourced testing can cost more due to third-party margins, leading to higher healthcare costs overall, regardless of payer.
In-house testing can help reduce cost to the healthcare system and patients with the right NGS test.
According to published costing analysis, it also becomes less expensive to run a panel, instead of 3 or more single biomarker tests
-

3) Shorter time to results—faster treatment initiation
Results from third-party labs can take weeks3—time that late-stage NSCLC patients don’t have to spare.
In-house testing with the Oncomine Dx Target Test is completed in as little as four days4, and returns a comprehensive report on relevant NSCLC biomarkers.
The ability to expedite the testing process enables a faster start of the right treatment for your patients.

Guidelines "strongly recommend" next-generation sequencing panel testing6
Next-generation sequencing (NGS) testing enables delivery of multiple biomarker results from one sample, minimizing the risk of depleting tissues and additional biopsies.
There are a number of biomarkers associated with approved therapies for NSCLC, with many others in clinical trials. In fact, 73%5 of all drugs in the oncology pipeline have associated biomarkers.
Today, NGS panel testing is the recommended approach to quickly assess patients because single-gene testing requires more tissue and time. The need for NGS will increase as new biomarkers are discovered.
But not all NGS tests are equal.

Tissue is the issue—why choosing the right NGS test is vital
Using NGS tests with larger tissue requirements may restrict many patients from receiving the full range of actionable biomarker results.
Insufficient sample can lead to test rejection or failure and therefore rebiopsies and treatment delays.
A comparison of two NSCLC FDA-approved companion diagnostic (CDx) tests reveals a significant difference in tissue requirements.
See additional data on tissue requirements from labs around the world. View Infographic »

Third-party labs—is sending out sustainable long term?
Outsourcing was a logical choice in the early days of biomarker testing.
The science was relatively new, it wasn’t reimbursable, and there were too few biomarkers to justify in-house testing. These former challenges are now becoming irrelevant.
Today, the science has matured, the technology is easy to implement, and more hospitals are now bringing NGS testing in-house to benefit patients.
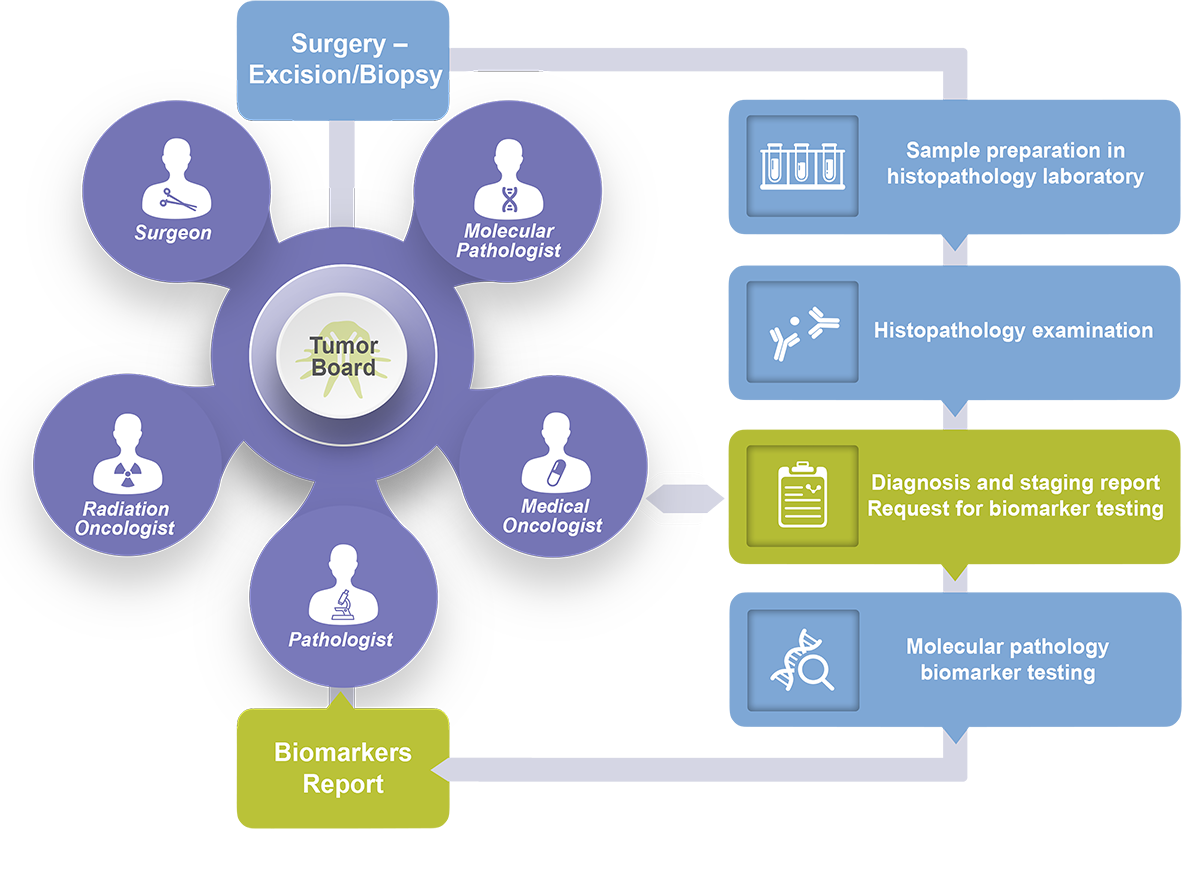
Improve care team coordination with in-house biomarker testing
Precision oncology is a medical practice that occurs at the local level, at the patient’s bedside and in interactions between local healthcare professionals including molecular pathologists. The flexibility to triage patient samples and to discuss in depth the findings at local tumor boards is key to providing the best and truly personalised care.
Implementing the NGS panel testing with Oncomine Dx Target Test will enable your care team to do just that.
Have questions? Ask one of our NGS sales consultants.
Multi-biomarker testing for more effective patient care is here today. If you or members of your care team have questions, our team is available to answer them.
References
- Tissue Requirements in NSCLC Patients: http://www.captodayonline.com/ngs-take-top-spot-cancer-biomarker-testing-broadens/
- Foundation Medicine's Testing Sample Requirements: https://www.foundationmedicine.com/genomic-testing/foundation-one-cdx
- Foundation Medicine's Testing Turnaround Time: https://www.foundationmedicine.com/genomic-testing/foundation-one-cdx
- Oncomine Dx Target Test Workflow: https://www.thermofisher.com/us/en/home/clinical/diagnostic-testing/condition-disease-diagnostics/oncology-diagnostics/oncomine-dx-target-test/oncomine-dx-target-test-us-only.html
- THE PERSONALISED MEDICINE REPORT; Personalised Medicine Coalition: http://www.personalizedmedicinecoalition.org/Userfiles/PMC-Corporate/file/The-Personalized-Medicine-Report1.pdf
- NCCN Guidelines Non-Small Cell Lung Cancer V6.2018
The Oncomine Platform software tools have moved.
The Oncomine Dx Target Test is For In Vitro Diagnostic Use.